However, there is some encouraging news for those with Alzheimers and their families.
Thehuman trialcomprised 1,795 participants with mild cognitive impairment from Japan, China, the U.S., and Europe.
Results show that the drug slowed progress of the disease by 27% compared with a placebo.

Photo: PictureDesignSwiss/Depositphotos
The drug, an intravenous antibody, binds to and removes these toxic proteins.
Biogens previous Alzheimers drug, Aduhelm, worked to do the samealthough it was not nearly as successful.
But other experts remain cautious to accept these conclusions fully.

Photo: postmodernstudio/Depositphotos
Other experts have suggested that the anti-amyloid treatment is only addressing part of the problem.
Therefore targeting a single target is not likely to produce large effect sizes.
Illustration of three stages of Alzheimer’s, a healthy neuron slowly dying from amyloid proteins.
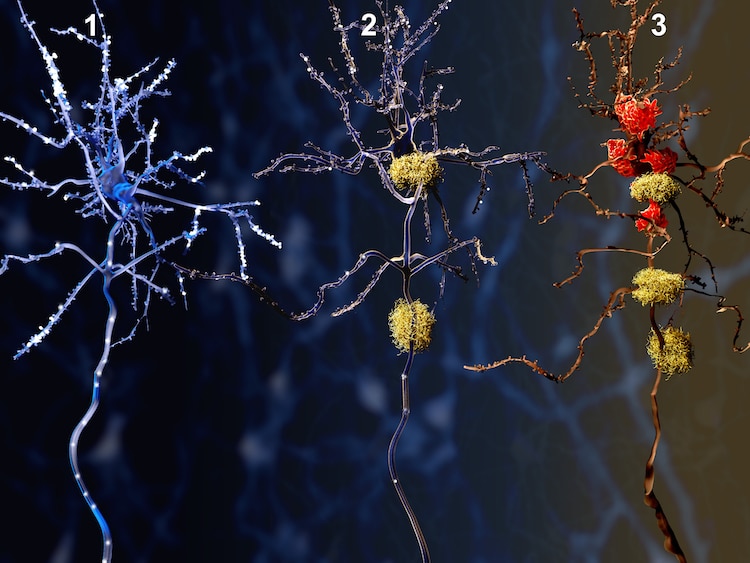
Illustration of three stages of Alzheimer’s, a healthy neuron slowly dying from amyloid proteins. Photo: animaxx3d/Depositphotos
Photo: IgorVetushko/Depositphotos

Photo: AndrewLozovyi/Depositphotos

Photo: Kubko/Depositphotos

Photo: grandbrothers/Depositphotos